Animal welfare is usually defined by reference to the adaptation efforts necessary for the animal to cope with its environment. Permanent adjustments to maintain the 'milieu int�rieur' (Bernard, 1878) within physiological limits despite variable environmental conditions are permitted by homeostatic mechanisms involving most physiological systems (Cannon, 1935). However, when the pressure from the environment becomes excessive, or in case of psychological threats, new defence mechanisms are initiated, collectively referred to as stress responses (Selye, 1973). All the above-mentioned pioneers of adaptation / stress physiology recognised that part of the stress response was the result of the emotional arousal elicited by environmental stimulations, and this aspect was further given more attention (Mason, 1971), so that the study of stress moved from the field of physiology to psychophysiology. This resulted in the integration of new concepts inherited from psychology, including coping that refers to the ability for the individual to control its environment and temperament that encompasses the individual variability of emotional processes (Lazarus, 1993). It was therefore recognised that the brain plays a central role in adaptation / stress mechanisms. The
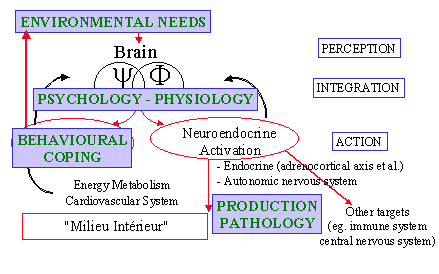
central nervous system collects from the external (via sensory organs) and internal environment the information necessary for the maintenance of homeostasis; it gives significance to this information in terms of danger or threat, as related to personal expectations, past experience and opportunities for control; finally, it initiates the adaptive responses, including behavioural adjustments, and neuroendocrine changes to meet the energy requirements for the behavioural response and to maintain homeostasis (Fig.1).
Figure1. General organization of adaptive responses
These concepts about adaptation and stress have long been shown to be operative for the analysis of the way farm animals deal with their environment (Dantzer and Morm�de, 1983). The 'five freedoms' elaborated by the Brambell Committee of the U.K. Parliament in 1965 and updated by the Farm Animal Welfare Council (1992), that define the obligations of humans to ensure the welfare of farm animals, can be seen as a formal expression of the various components of this integrative psychobiological view of adaptation and stress (Fig.2):
Freedom from hunger and thirst refers to the physiological requirements,
Freedom from discomfort refers to the environmental needs,
Freedom from pain, injury and disease refers to the pathological consequences of an adverse environment,
Freedom to express normal behaviour refers to the importance of behavioural coping mechanisms for a successful adaptation,
Freedom from fear and stress refers to the psychological component of adaptation.
Figure 2. Adaptive responses and the five freedoms
|
Several measures can be used to evaluate the welfare state of the animals, including behaviour, biological functions related to stress physiology, as well as zootechnical and pathological data. These evaluations should be based on multicriteria approaches, since no single measure can unequivocally be related to the level of welfare. Among those, neuroendocrine parameters are probably the most widely used, since corticosteroid hormone secretion by the adrenal cortex has been equated with stress level since the very beginning of the stress concept, and their measurement in plasma is relatively easy. Indeed, circulating levels of corticosteroid hormones are exquisitely sensitive to a wide range of stimuli including low level of emotional activation such as induced by novel environment exposure or social encounter. A large body of experimental data also shows that the hypothalamic-pituitary-adrenocortical (HPA) axis activity is related to the behavioural coping strategy, with active attempts to keep control over the situation being associated with a preferential activation of the sympathetic nervous system (SNS) whereas passive / submissive behaviours are associated with a higher secretion of corticosteroid hormones (Henry and Stephens, 1977).
However, it must be kept in mind that the HPA axis and the SNS are primarily involved in the regulation of numerous homeostatic processes, including energy metabolism, independently of the stress state. For example, the activity of the HPA axis is strongly influenced by the diurnal cycle, with a higher activity at the beginning of the behaviourally active period, at lights on for diurnal animals and at lights off for nocturnal animals. These diurnal changes are the result of the co-ordinated influence of the light cycle per se and of metabolic factors. There is also experimental evidence that plasma corticosteroid levels and catecholamines secretion are strongly influenced by the feeding regimen, although this aspect has not been investigated in great detail in farm animals. It is therefore important to be able to sort out the various influences impinging upon the physiological systems under study, since physiological adjustments and psychobiological influences are usually intermingled in the global stress response measured by circulating stress hormone levels.
Another factor to be taken into consideration is the exquisite sensitivity of the neuroendocrine systems to procedural factors such as handling and venous puncture for blood sampling, so that specific approaches have been developed to minimise the influence of the experimenter (e.g. saliva sampling for the assay of corticosteroids, telemetric monitoring of cardiac activity to evaluate SNS function). We are evaluating in pigs and other species the interest of measuring urinary levels of corticosteroids and catecholamines as indices of HPA axis and SNS function respectively. This approach has several potential advantages as compared to other methods of investigation (Hay et al., 2000):
urine can be collected with minimal disturbance to the animals; therefore it does not introduce an experimental bias and it is convenient for field studies,
the excretion products in urine sum up over several hours (i.e., since the last urination or collection time) so that hormone concentrations in urine are independent of the rapid variations of hormone release (related either to the pulsatility of neuroendocrine functioning or to procedure-related disturbances) and are therefore more integrative than plasma or saliva concentrations,
both the HPA axis and SNS activity can be evaluated simultaneously, corticosteroids and catecholamines being measured in the same sample by specific HPLC procedures after solid phase extraction.
It is therefore expected that the monitoring of hormone levels in urine will allow an easier detection of long-term variations in HPA axis and SNS basal activity, as induced by chronic stressful situations or as related to different behavioural reactivity to emotional stimuli.
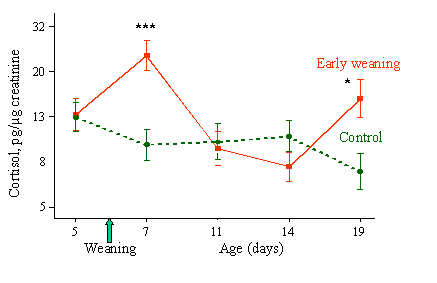
Recent data obtained in the study of early weaning (6 days) in pigs illustrate the interest of such a psychoendocrine approach for the study of animal welfare. As shown in figure 3, early weaning strongly reduced the growth rate of piglets, mostly because of a low ingestion of dry food during the first days after weaning. Urinary cortisol levels increased the day after weaning and rapidly returned to control levels afterwards (Fig. 4). Indeed, weaning has already been shown to increase cortisol secretion using plasma sampling, even at later ages (Carroll et al., 1998; Dantzer and Morm�de, 1981; Kanitz et al., 1998), and from this result, it could be concluded that early weaning induces a short-lasting stress, not much different from classical weaning at 3-5 weeks.
Figure 3. Early weaning in pigs - effect on body growth
Figure 4. Early weaning in pigs - urinary cortisol
|
The picture is however different when considering the changes in catecholamine excretion (Fig.5). Noradrenaline concentration in urine showed a sharp and persistent decline and did not return to control levels until day 19, 2 weeks after weaning. Adrenaline concentration in urine remained stable until day 11, but therefore dropped down to very low levels until the end of the experiment. These results can be explained by the role played by catecholamines in energy regulation. Noradrenaline is mainly calorigenic through fat mobilisation and activation of brown adipose tissue metabolism. Thus, the activity of the noradrenergic system is tightly coupled to energy balance. Therefore the drop of noradrenaline likely reflects an energy saving mechanism related to the shortage of energy intake (Young and Landsberg, 1977). Indeed early weaned piglets spent more time under the infrared lamp and huddled together more than control animals, these thermoregulatory behaviours showing the deficit of metabolic thermogenesis.
On the other hand, adrenaline is mainly involved in the maintenance of blood glucose levels via glycolysis and its late drop may be related to the exhaustion of glycogen stores (Young et al., 1984). Therefore, these very large changes in catecholamine excretion show that adaptive mechanisms are considerably taxed by early weaning, although we have no comparative data at the present time for classical weaning. It is worth noting that the intensity of the challenge to homeostatic mechanisms by early weaning was not revealed by the measurement of adrenocortical axis activity. Indeed, the transient increase of cortisol excretion could be related more to the psychological consequences of weaning (rupture of the mother-young link) but does not give a complete picture of the adaptation needs. This strengthens the idea that focusing on a sole physiological system gives an incomplete picture of the challenge imposed to the animals by their environment.
Figure 5. Early weaning in pigs - effect in urinary catecholamines
Figure 6. Concentration of cortisol and catecholamines in urine from Meishan and Large White lactating sows and their F1 hybrids
A large invidual variation can be observed in behavioural and neuroendocrine responses to environmental challenges. A large body of experimental evidence obtained mostly in experimental animals shows that these differences arise from the complex interplay of genetic factors, environmental influences during development (gestation and neonatal period), as well as learning processes from past experience. In farm animals, general behavioural reactivity profiles have been described, similar to temperaments in humans and experimental animals (Ramos and Morm�de, 1997), as well as specific behavioural reactivity traits, like fear of humans (Lankin, 1997), that are important for their welfare. Large differences can also be found in the neuroendocrine profile (Hay and Morm�de, 1998; Fig.6).
The influence of genetic factors in the shaping of these profiles of reactivity is overwhelming and well documented (D�saut�s et al., 1997, 1999), and a few studies have dealt with the influence of early environmental influences and learning processes. Furthermore, variations in behavioural and neuroendocrine traits are also related to production traits (D�saut�s et al., 1997). Indeed, as stated previously, neuroendocrine systems reactive to stress are primarily involved in metabolic regulations, and it is well established for example that an hyperactive HPA axis promotes the production of fat (e.g. pigs from the Meishan breed). More recently we also found that meat quality was related to the neuroendocrine profile as measured in urine collected after slaughter (unpublished results). Current molecular genetic studies should give some clues to the biological mechanisms involved in these individual differences of behavioural and neuroendocrine reactivity.
Future improvements of animal welfare should focus on the ways animals react to their environment and not only on changes in the environment, or at least they should take into account the individual diversity of adaptive responses and psychoendocrine reactivity. These reactivity traits can be selected for a better adaptation to environmental constraints, and the shaping of reactivity can also be obtained by early manipulation of the animals or specific training. Available data show that production output and product quality may also be concomitantly increased. Such a goal will be reached by a larger appraisal of animal - environment interactions, combining behavioural and biological approaches, and with the development of new strategies to evaluate more comprehensively the psychoendocrine mechanisms of adaptation.
REFERENCES
Bernard C (1878) Introduction � la M�decine Exp�rimentale. Paris, Garnier-Flammarion, 1966.
Carroll JA, Veum TL, Matteri RL (1998) Endocrine responses to weaning and changes in post-weaning diet in the young pig. Dom.Anim.Endocrinol. 15:183-198.
Cannon WB (1935) Stresses and strains of homeostasis. Amer.J.Med.Sci. 189:1-14.
Dantzer R, Morm�de P (1981) Influence du mode d'�levage sur le comportement et l'activit� hypophyso-corticosurr�nalienne du porcelet. Reprod.Nutr.Dev. 21:661-670.
Dantzer R, Morm�de P (1983) Stress in farm animals: a need for reevaluation. J.Anim.Sci. 57 :6-18.
D�saut�s C, Bidanel JP, Morm�de P (1997) Genetic study of behavioral and pituitary-adrenocortical reactivity in response to an environmental challenge in pigs. Physiol.Behav. 62:337-345.
D�saut�s C, Sarrieau A, Caritez JC, Morm�de P (1999) Behavior and pituitary-adrenal function in Large White and Meishan pigs. Dom.Anim.Endocrinol. 16:193-205.
Farm Animal Welfare Council (1992) FAWC updates the five freedoms. Vet.Rec. 131:357.
Hay M, Morm�de P (1998) Urinary excretion of catecholamines, cortisol and their metabolites in Meishan and Large White sows: validation as a non invasive and integrative assessment of adrenocortical and sympathetic activity. Vet.Res. 29:119-128.
Hay M, Meunier-Salaün MC, Brulaud F, Monnier M, and Morm�de P. (2000) Assessment of hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity in pregnant sows through the measurement of glucocorticoids and catecholamines in urine. J.Anim.Sci. 78:420-428.
Henry JP, Stephens PM (1977) Stress, health and the social environment. A sociobiologic approach to medicine. Spinger Verlag, New York.
Kanitz E, Manteuffel G, Otten W (1998) Effects of weaning and restraint stress on glucocorticoid receptor binding capacity in limbic areas of domestic pigs. Brain Res. 804:311-315.
Lazarus RS (1993) From psychological stress to the emotions: a history of changing outlooks. Annu.Rev.Psychol. 44:1-21.
Mason JW (1971) A re-evaluation of the concept of 'non-specificity' in stress theory. J.Psychiatr.Res. 8:323-333.
Ramos A, Morm�de P (1998) Stress and emotionality : a multidimensional and genetic approach. Neurosci.Biobehav.Rev. 22 :33-57.
Lankin V (1997) Factors of diversity of domestic behavior in sheep. Genet.Sel.Evol. 29 :73-92.
Selye H (1973) The evolution of the stress concept. Amer.Scient. 61:692-699.
Young JB, Landsberg L (1977) Suppression of sympathetic nervous system during fasting. Science 186:1473-1475.
Young JB, Rosa RM, Landsberg L (1984) Dissociation of sympathetic nervous system and adrenal medullary responses. Am. J. Physiol. 247:E35-E40.
|